Abstract
Introduction
Chimeric antigen receptor T (CAR T)-cell therapy has substantial activity in relapsed lymphoid malignancies. However, it is associated with important complications, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Venous thromboembolic events (VTEs) following CAR T-cells have been reported with variable incidence. Risk factors for the development of VTEs following CAR T-cell therapy are largely unknown, including whether the CAR T-cell construct is associated with increased VTEs.
Methods
We retrospectively evaluated patients treated at our institution who received CAR T-cell therapy for non-Hodgkin lymphoma between May 2018 and January 2022. Baseline characteristics and outcomes were collected. VTEs included deep venous thrombosis and pulmonary embolism. The cumulative incidence (CI) of VTE was calculated with death as competing risk and comparisons between groups were done with Gray test. Univariate comparisons of baseline parameters were performed with Fisher's exact test evaluating the following covariates: age (older or younger than 70 years), LDH and C-reactive protein levels, ECOG performance status, revised IPI, BMI, personal history of VTE, and stage. Due to the low number of VTEs, we did not attempt multivariate modeling based on baseline characteristics. All analyses were performed using R. This study was approved by our Institutional Review Board.
Results
We identified 95 patients treated with CAR T-cells between May 2018 and January 2022. The majority had diffuse large B-cell lymphoma (DLBCL) (88, 93%). Baseline characteristics are listed in Table 1. Prior history of VTE occurred in 16 patients and 27 received at least one dose of therapeutic or prophylactic anticoagulation post-CAR T infusion prior to developing VTE. Most patients had central venous access during their hospitalization for CAR T cell therapy (n = 91). A total of 56 patients received axicabtagene ciloleucel (58.9%), 30 patients received tisagenlecleucel (31.6%), 5 patients received brexucabtagene autoleucel (5.3%), and 4 received lisocabtagene maraleucel (4.2%).
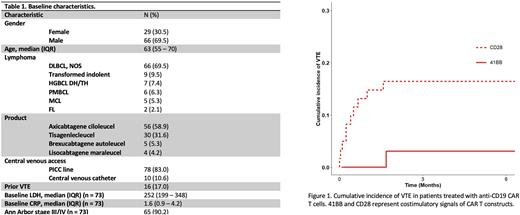
Post-CAR T VTE occurred in 13 patients (14%); median time to VTE (IQR) was 15 days (7-48), with a 100-day cumulative incidence of 12%. All VTEs were DVTs and the majority were PICC line-associated (10, 77%). The incidence of VTE was higher for patients treated with axicabtagene ciloleucel or brexucabtagene autoleucel in contrast to 41BB-containing constructs (16% vs. 3%, p = 0.03) (Figure 1). Among baseline characteristics, a prior history of VTE was associated with increased risk of subsequent VTE (39% vs. 13% without prior VTE, p = 0.04). The 100-day CI of VTE for patients with and without CRS were 15% and 4% (p = 0.09), while the 100-day CI of patients with and without ICANS were 18% and 7% (p = 0.09).
Subgroup analysis of patients treated with CD3ζ/CD28 signaling showed that 100-day VTE CI was higher among patients with a prior history of VTE (33% vs. 12%, p = 0.03) and those with BMI of ≥ 30 (38% vs. 11%, p = 0.03). We did not observe an increased incidence of VTE in patients with baseline ECOG performance status ≥ 2, stage III/IV disease, elevated baseline lactate dehydrogenase or C-reactive protein.
After a median follow up of 24 months (IQR 13-36), the median progression free survival (PFS) was 7 months (95% CI = 4-12) while the median overall survival (OS) was 14 months (95% CI = 11-19), there were no statistically significant differences in PFS or OS among patients with and without VTE.
Conclusions VTEs are an emerging toxicity associated with CAR T cell therapy. We show that increased VTE incidence at 100 days is associated with the use of CD3ζ/CD28 CAR T-cells. This difference may correlate with differing degrees of systemic inflammatory response, as previous reports have detailed higher incidence of high-grade CRS with axicabtagene ciloleucel vs. tisagenlecleucel, and other construct-specific factors may further increase VTE risk in patients with established VTE risk factors (e.g. history of VTE and obesity). We also observed that a large proportion of post CAR T VTEs corresponded to line-associated DVTs. Taken together, these findings may help identify patients at increased risk of VTE after CAR T-cell therapy and potentially guide decisions on surveillance, choice of central access, or prophylactic anticoagulation.
Disclosures
Hamilton:Nkarta: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Equilium: Membership on an entity's Board of Directors or advisory committees. Winter:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seagen, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria. Jagadeesh:ATARA Biotherapeutics: Research Funding; Seagen: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees; Trillium Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Debio pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; Regeneron Pharmaceuticals, Inc.: Research Funding; AstraZeneca: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sauter:Genzyme/Sanofi: Other: PI; BMS: Other: PI; Gamida Cell: Consultancy; Precision Biosciences: Other: PI; CSL Behring: Consultancy; Karyopharm Therapeutics Inc.: Consultancy; Kite Pharma Inc.: Consultancy; Ono Pharmaceuticals: Consultancy. Hill:Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding. Caimi:Janssen: Consultancy; MEI Pharma: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Research Funding; GenMab: Honoraria; Incyte: Consultancy; Novartis: Consultancy; BMS: Honoraria; Genentech: Consultancy; Kite: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal